
These inner electrons shield the outer electron from the nucleus, thereby lessening the electrostatic attraction.Īs the electrostatic attraction between the outer electron and the nucleus is weaker in a potassium atom than in a lithium atom, less energy will be required to overcome the electrostatic attraction and remove the outer electron. In addition, there are many more electrons between the outermost electron and the nucleus in a potassium atom than there are in a lithium atom. This is due in part to the fact that the outer electron of a potassium atom is farther away from the positively charged nucleus. The outermost electron of a potassium atom is electrostatically attracted to the positively charged nucleus but to a lesser degree than the outer electron of a lithium atom. Two electrons are in the first electron shell, eight electrons are in the second and third electron shells, and one electron is in the fourth electron shell. So let’s take a look at an atom that’s larger than lithium.

Atomic radius is a quantity that defines the size of an atom. To answer this question, we need to determine how the atomic radius affects the ionization energy. If we supply the ionization energy to the atom, the electrostatic attraction between the electron and the nucleus can be overcome and the electron can be completely removed from the atom. This electron is electrostatically attracted to the positively charged nucleus. The most loosely bound electron in this atom is the electron found in the outermost electron shell. Two are in the first electron shell and one is in the second electron shell.
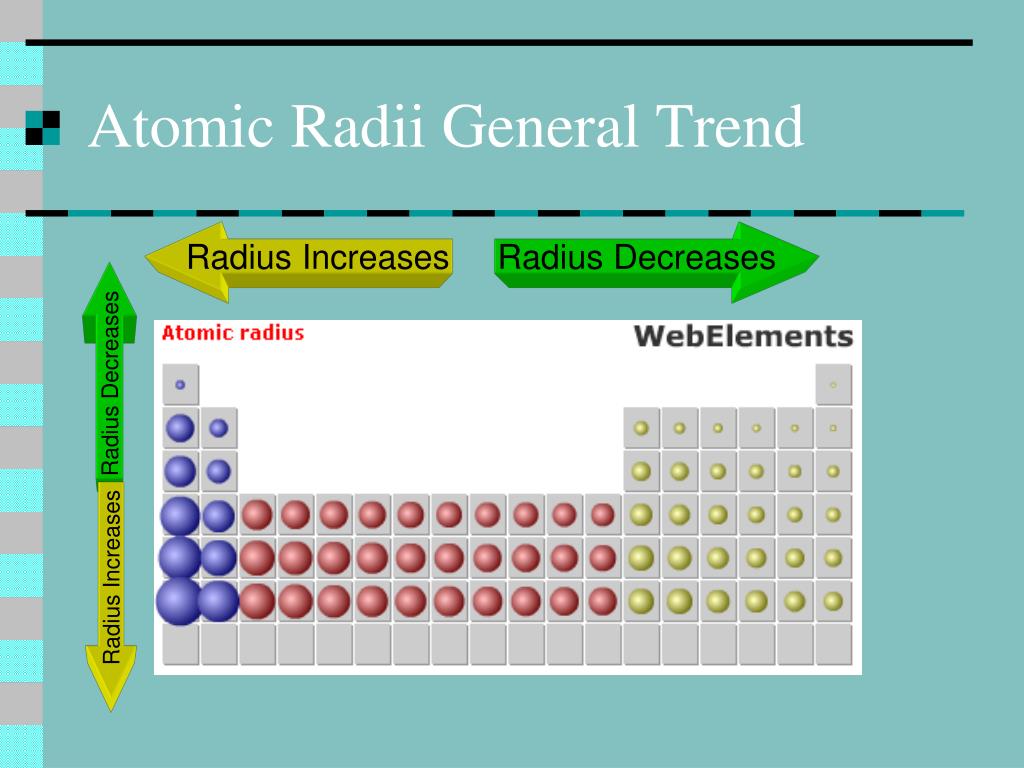
Let’s consider an atom of lithium, which has three electrons. When we talk about ionization energy, we are most often referring to the first ionization energy, which is the amount of energy required to remove the most loosely bound electron completely from an isolated gaseous atom. Or (D) the atomic radius does not affect ionization energy at all. (C) Decreasing the atomic radius leads to a lower ionization energy. (B) Increasing the atomic radius leads to a higher ionization energy.
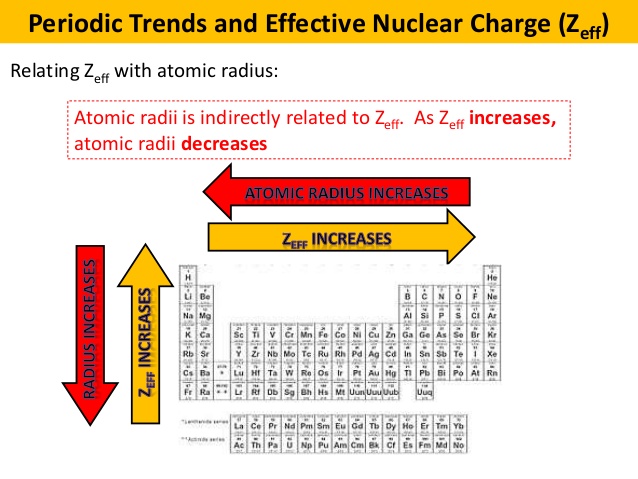
How does the atomic radius affect the ionization energy? (A) Decreasing the atomic radius leads to a higher ionization energy.


 0 kommentar(er)
0 kommentar(er)
